A medical scandal in the making
Julia Belluz on flawed regulations and medical devices
Shutterstock
Share
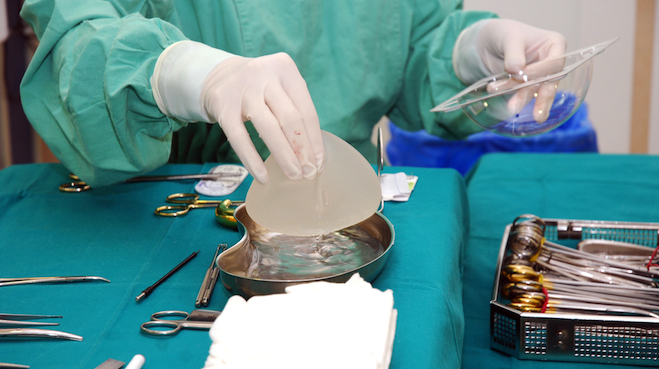
In France, a global medical scandal continues to brew after three years in the public eye. For up to a decade, French-made Poly Implant Prosthesis (PIP) breast implants were being placed in women’s chests. In 2010, the medical community found that, despite being approved by regulators, the implants were actually filled with the kind of silicone better suited for mattresses than women’s bodies. They were also at a higher risk of rupturing, and some academics worried aloud that they could adversely effect the development of a fetus.
European regulators were fingered for letting the implants slip through to market, and the varied decisions by countries to authorize them for sale (the U.S. and Canada didn’t, while France and England did) demonstrated a clear lack of harmonization in medical device regulation.
Now, the PIP executives at the centre of the scandal are waiting to find out whether they’ll be thrown in jail. But the problem of flawed medical-device regulation won’t end with their trial—nor is it Europe’s alone. And as the market for and prevalence of these devices continues to grow, they represent a science-ish scandal in the making.
The term ‘medical devices’ includes any medical product that does not accomplish its raison d’être through a chemical process—breast implants, hip replacements, surgical equipment, even tongue depressors. Unlike drugs, there are no international registries where data for these devices can be shared, and poor post-marketing surveillance to monitor what happens with them after they hit the market.
Here in Canada, an uncoordinated approach to devices leaves approval processes and pricing all over the map. No one knows exactly how these devices enter the system after Health Canada approves them because we have a “bottom-up” system in place, whereby individual surgeons or nurses can introduce new machinery in their clinics or hospitals with only institutional oversight.
If you thought drug regulation left much to be desired, international device regulation is even patchier, according to health-care insiders. Just read up on faulty vaginal mesh, or the infamous DePuy ASR hip replacement recall. Dr. Ratika Parkash, a physician-academic with Dalhousie University in Halifax, studied recalled defibrillator wiring, the Sprint Fidelis Leads. These tiny wires connect defibrillators to the heart and were implanted in nearly 250,000 people around the world (and some 6,000 Canadians) before it was revealed they broke much more easily than other models, which meant the defibrillator couldn’t perform its life-saving shocks to the heart.
“There was a major uptake for this device right away without a lot of in-human data,” said Dr. Parkash, explaining that the Fidelis Lead debacle was an example of the inferior pre-marketing system for devices. “For a new drug, there are large phase-three clinical trials that are required in a large number of patients with robust endpoints. For medical devices, there’s not a lot required to get them approved.”
Health Canada told Science-ish that all devices imported or sold in Canada must meet fundamental safety, labelling and effectiveness requirements, and that manufacturers of higher risk medical devices must seek licenses with “objective evidence.” But Dr. Parkash is not the only one to point out that the evidence manufacturers give to regulators isn’t very robust. In this BMJ analysis, key thinkers on medical regulation wrote, “Many medical devices are produced by small specialist companies that lack the finance and experience to conduct adequate research, and the relatively short ‘market life’ of many devices also militates against continuing research.” They added: “Adoption of the new device or procedure is typically driven by marketing and the enthusiasm of clinicians, rather than by evidence.”
Part of the problem, of course, is that it’s much more difficult to systematically study the impact of devices. “There are so many variables,” said Dr. Shady Ashamalla, assistant professor in surgery at the University of Toronto. “It makes it extremely difficult for the science to have true validity.” If you sew a patient up after surgery using a new technique, for example, and a few days later the stitch falls apart, it’s difficult to know whether it was the material used, the patient or the technique, he explained.
At Dr. Ashamalla’s hospital, only devices that cost more than $25,000 go through a competitive bidding process to gain entry into the system. Otherwise, it’s up to clinicians to initiate the process of hospital purchasing, and they rely on representatives from device companies to teach them about new technology. While Dr. Ashamalla said this informal process speeds up innovation, he added: “There shouldn’t be an entitlement that whatever the company makes we will use. We want to work together so that the best product is the one we go with but we don’t want to feel committed.”
Indeed, McMaster University researcher Dr. Brian Haynes worries these relationships can skew clinical judgements. He pointed out that the diabetes clinic where he works is like a “honey pot” for device reps who often give away free glucose meters to help patients self-monitor their glucose levels. But they don’t give away the disposable test-strips that are needed for use in the meters. If patients check their levels three times per day, they need to buy about $90 worth of strips every month to go with their free machines. Yet, the systematic reviews of these machines show they have little effect on helping patients control their blood glucose levels, and their use has even been linked with higher rates of depression.
“When patients are tested,” Dr. Haynes told Science-ish, “it’s more often the case than not that (self-monitoring glucose meters) have not been useful in terms of improving patient health, or at least cost much more than anyone could have imagined for a miniscule benefit. And yet, most devices receive no formal testing for whether patients are better off.”
What’s worse, there’s little by way of comparative-effectiveness research, or the kinds of studies that look at how devices fare when compared with each other, and whether they actually improve a person’s health. This means clinicians don’t have the kind of evidence they need to make well-informed decisions about whether to use a device or not. “At least with drugs, you can’t prescribe until that drug passes an acid test,” he added, “but that’s not true for a device that might be used for warts or for zapping lesions on the cervix.”
Europe is working on tightening up controls around medical devices in response to the PIP scandal, and Health Canada pointed to its recently published Regulatory Roadmap for Health Products and Food, which includes a rather amorphous vision for reforms to how devices are handled here such as “supplementing” post-marketing surveillance and “other smaller policy changes required for international alignment.”
For now, there’s a group in Canada—known as the Pan-Canadian Health Technology Collaborative—that’s about to start studying exactly how these devices enter the system. “We recognize now we don’t know who is making the decisions about purchasing a piece of equipment for one institution,” said Dr. Michelle Mujoomdar of CADTH, one of the people on the study.
“The volume of devices entering the system is high. We have a lot of information about drugs coming to the market but on the non-drug side, it’s much more diffuse.”
Science-ish is a joint project of Maclean’s, the Medical Post and the McMaster Health Forum. Julia Belluz is the associate editor at the Medical Post. Got a tip? Seen something that’s Science-ish? Message her at [email protected] or on Twitter @juliaoftoronto