What you don’t know about a leading morning-sickness drug
A top researcher has been sworn to secrecy about a medication prescribed to half of pregnant women in the country
TORONTO, ON – APRIL 22: Dr. Navindra Persaud poses at the St. Michael’s Family Practice Clinic, April 22, 2015. Persaud struggled getting information about a popular morning sickness drug sparked a years long battle with Health Canada. (Andrew Francis Wallace/Toronto Star/Getty Images)
Share

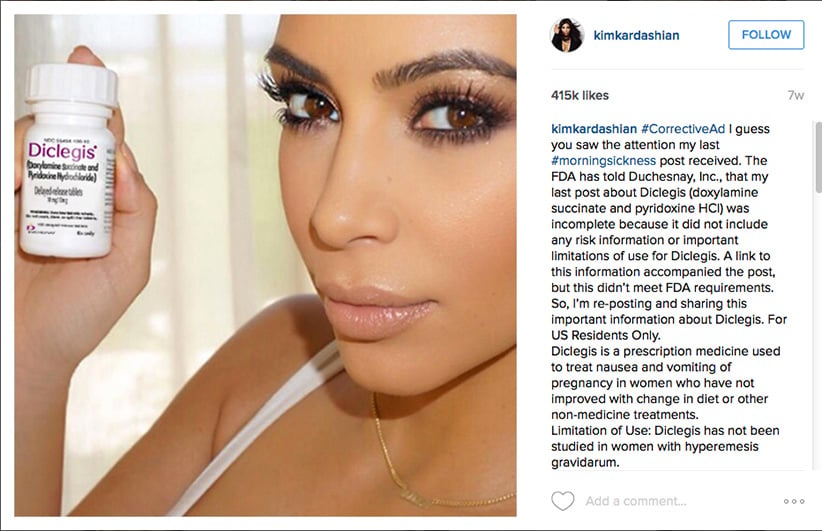
Last August, Toronto family physician Nav Persaud watched with amazement—and some frustration—as Kim Kardashian accomplished with one Instagram post what he had been trying to do for years: Put klieg lights on a prescription drug for morning sickness in pregnancy. Persaud, a drug researcher at Toronto’s St. Michael’s Hospital, had been investigating Diclectin, a time-release tablet that combines pyridoxine (vitamin B6) and the antihistamine doxylamine manufactured by Duchesnay Inc. in Blainville, Que. The medication, on the market in Canada since 1983, and the only prescription drug approved by Health Canada to treat the nausea and vomiting of pregnancy (NVP), is a staple in this country; it is prescribed in one of two live births. Its popularity has been paved by assurances and positive studies from what was once an unimpeachable source: Toronto’s Hospital for Sick Children’s Motherisk unit, the front line for information for doctors and women on the safety of drugs, toxins and chemicals in pregnancy and lactation. Physician and clinician Gideon Koren, who founded Motherisk in 1985, was a vocal proponent of the drug, while also a paid consultant to Duchesnay.
Persaud’s research into a drug whose controversial history includes birth-defect litigation and removal from international markets came to a different conclusion. A 2013 study written with St. Michael’s colleagues, “Re-analysis of safety data supporting doxylamine use for nausea and vomiting of pregnancy,” published in the American Journal of Perinatology, found errors in the 1997 Motherisk metastudy that buttressed the Society of Obstetricians and Gynaecologists of Canada’s 2002 recommendation of the drug as the only therapy for NVP. The St. Mike’s researchers claimed data were misattributed, and questioned the claims regarding the drug’s safety. In a 2014 commentary written with many of the same authors in the Journal of Obstetrics and Gynaecology Canada, Persaud argued that there is “stronger support for the safety” of non-prescription vitamin B6 alone; the authors contend Diclectin’s benefits are overstated and that prescribing it as a first-line treatment for NVP should be reconsidered. Both studies received a smattering of media coverage, but no further action.
So when Kardashian took to social media in July 2015 to shill for Diclegis, the same compound as Diclectin (approved by the U.S. Food and Drug Administration in 2013), Persaud, with the rest of the world, couldn’t help but notice. “OMG. Have you heard about this?” the reality-TV star gushed to 42 million followers with a photo of herself holding the bottle. “As you guys know my #morningsickness has been pretty bad,” she continued, before making assurances the drug had been “studied” and there was no increased risk to her baby by taking it: “I’m so excited and happy with my results that I’m partnering with [drug maker] Duchesnay USA to raise awareness about treating morning sickness.”
Persaud watched with interest as the FDA then fired off a warning letter to Duchesnay USA, posted on the FDA website on Aug. 7, stating that the ad violated federal regulations in several areas and ordering the post be taken down—a move that only summoned more publicity for the drug. “These violations are concerning from a public health perspective, because they suggest that Diclegis is safer than has been demonstrated,” wrote Robert Dean of the FDA’s Office of Prescription Drug Promotion. The regulatory agency objected to claims being made about the drug’s efficacy, and to the lack of reference to risks that include use by women with asthma, as well as some problems with the eyes, stomach and bladder. Nor was there mention of side effects such as serious drowsiness. (Women should avoid driving until “cleared to do so by their health care provider,” the FDA warns.) The FDA also ordered corrective measures, which would include Kardashian issuing a second social media post in late August listing the drug’s risks and links to websites outlining side effects.
The FDA’s demands for fuller disclosure about Diclegis stood in stark contrast to the months of negotiations Persaud was having with Health Canada, this country’s drug regulator, in his quest for data about Diclectin. He’d made the request under “Vanessa’s Law,” which went into force in November 2014 to provide greater access to drug clinical data. The government agency agreed to hand over 35,000 pages, but only if Persaud signed a confidentiality agreement that would muzzle him from speaking about the data or referencing it publicly; he’d face legal action if he did. He’d also have to destroy all documents after review and notify Health Canada in writing when he did. Agreeing to the terms was difficult, Persaud told Maclean’s last week: “I wasn’t sure what Health Canada had and what they would disclose. I thought that if I signed the agreement, [then I] could see what was there.”
After reviewing the data received in mid-September, Persaud is circumspect: “What I can say is that I’m concerned that the medication is not effective at all,” he says. “But after receiving advice, I can’t discuss why I now believe that the medication is not effective. I know this is an absurd situation. It’s going to be frustrating for your readers for me to make these vague statements, but that’s the situation I’m in.”
The confusion is compounded by ongoing questions about Motherisk research and former director Koren. Last year, as the result of a Toronto Star investigation, the unit came under fire for inaccuracies in its drug and alcohol hair-testing program, analysis routinely used in criminal and child welfare cases. Last week, SickKids’ CEO, Dr. Michael Apkon, apologized for “unacceptable” practices in that program and “to children, families and organizations who feel that they may have been impacted in some negative way.”
The Toronto Star investigation also found Koren’s financial relationship with Duchesnay was not always disclosed publicly. Last week, this took a new turn when the hospital released findings from its own ongoing internal review of Motherisk that revealed that, unbeknownst to SickKids until late 2014, Koren had created the Research Leadership for Better Pharmacotherapy during Pregnancy and Lactation to fund some of his research; this was “an aggregate” of unrestricted funds “donated by a variety of individuals and organizations to be used at the discretion of Dr. Koren.” The primary donor in recent years was Duchesnay.
The cloaked funding source is but the latest twist in the story of a drug whose 60-year history tracks the changes in attitudes toward medication taken in pregnancy—a trajectory that runs from lack of concern in the 1950s, to hypervigilence following the thalidomide tragedy of the ’60s, to a normalization that sees Kim Kardashian promote a morning-sickness drug much as she once endorsed shape-wear. That divided opinion still surrounds a drug taken by millions of pregnant women is unacceptable, of course. The confusion can be traced to the blurring of lines between medical research and corporate funding, industry-researcher alliances and how institutional clout—and high-profile endorsement—shape medical and public opinion.

Persaud’s questions about Diclectin began to percolate around 2011. Like many doctors, he prescribed the drug routinely for NVP, a condition that occurs in more than half of pregnancies. As he looked at the sources of information, many from Motherisk, his prescription stance changed: He gave it only to women who asked for it, even though he didn’t think it was effective, or that there was proof that the benefits outweighed the risks.
Persaud notes there’s not a large increased risk associated with Diclectin, with two caveats: “While the overall risk of malformations is not increased, there may be a slight increase in certain malformations, with conflicting evidence linking the drug to pyloric stenosis [an uncommon condition that affects the gastrointestinal tract during infancy],” he says. “The second is that, though most of the research is focused on malformations, some studies suggest there’s an association with a type of leukemia in children that wouldn’t be picked up when looking for birth defects. There’s not a clear indication it causes problems, just potential.”
Duchesnay insists Diclectin is safe, referring to it as “the most studied medicine for use during pregnancy,” on its website. The “safety of Diclectin has been repeatedly proven by 16 cohort studies, two meta-analyses,” as well as numerous other studies, company spokesman Ron Vaillancourt told Maclean’s in an email.
Persaud’s prescribing stance changed again recently, before he received the Health Canada data: “I can’t justify writing a prescription, given a lack of evidence that the drug is more effective than a placebo or vitamin B6 alone.” Efficacy is a salient point, given the drug’s potential side effects, its cost ($174 for 100 tablets, or $6.96 for the usual four-a-day dose, according to a Toronto pharmacy), and the fact that it’s covered by publicly funded provincial drug plans.
Examining whether or not Diclectin actually works is a marked departure from the decades-long focus on its safety, resulting from a similar compound being the centrepiece of birth-defect lawsuits in the U.S. That history begins with the drug Bendectin, then produced by Richardson-Merrell Inc. and approved in 1956 by the FDA for morning sickness. In its original formulation, Bendectin contained three ingredients: doxylamine succinate (an antihistamine), pyridoxine hydrochloride (vitamin B6) and dicyclomine hydrochloride (an antispasmodic). It became a leading treatment for NVP in the U.S., Canada, as well as internationally under different brand names.
In 1976, the drug was reformulated, with dicyclomine hydrochloride removed. The FDA website, as well as the “history of Bendectin” on Duchesnay’s website, reports that testing indicated dicyclomine hydrochloride offered no benefit for treating morning sickness. Other sources since then, however, link the compound to possible malformations; the 2013 Clinical Pharmacology During Pregnancy states that dicyclomine hydrochloride has “been associated with congenital malformations when used in combination with the antihistamine doxylamine; however, findings of teratogenicity [the capability of producing birth defects] have been inconsistent.”
In 1977, the first of some 2,000 U.S. claimants came forward, alleging the drug was responsible for birth defects, including limb malformations. (The overall rate of all malformations in live births is five per cent.) The lawsuits exposed the fact that research into Bendectin was scant, says lawyer Michael D. Green, author of the 392-page Bendectin and Birth Defects: The Challenges of Mass Toxic Substances Litigation. Green, a professor of law at Wake Forest University, told Maclean’s the drug was introduced when it was believed drugs didn’t cross the placental barrier. Thalidomide hadn’t come to market; mandatory registered clinical trials to test drug safety and efficacy were not routine. Some information unearthed in the early cases—including a letter from Merrell Dow Pharmaceuticals to a British researcher offering to endow an institute in his name if his research proved useful—played into plaintiffs’ hands, he says. “In absence of the fairly exonerative evidence that developed later, the plaintiffs had early successes.”
In time, federal judges shut down all the Bendectin litigation through a variety of procedural means, with verdicts in favour of plaintiffs later overturned. “Many judges have made it clear that they will no longer allow Bendectin cases in their courts,” says Green. But the ligitation did lead to study after study on the drug’s safety. “Bendectin was a case of litigation driving the science,” says Green.
Barry Nace, a Washington, D.C.-based lawyer who specializes in medical-malpractice suits, remains convinced to this day that Bendectin caused birth defects. “It wasn’t as vicious as thalidomide, but it had the same kind of effect,” he says. Nace won one of the first Bendectin cases in the U.S. in the early 1980s; he would act for plaintiffs in more than 200 cases. Bendectin’s litigation history was coloured by politics and the mishandling of several high-profile cases, he says. Some 30 years later, the topic remains unresolved: Green says he’d advise his daughter to take the drug if needed; Nace is opposed. They both agree on the lack of efficacy data. Merrell Dow’s own studies provided conflicting results, Nace claims: “One said it worked; one said it didn’t.”
In 1983, submerged in lawsuits, Merrell Dow voluntarily removed Bendectin from the worldwide markets, citing financial–not safety–concerns.
Canada was the lone country to continue to make pyridoxine-doxylamine available in a generic version produced by Quebec-based Laboratoires Duchesnay Inc. There is no indication additional studies were required for approval. Health Canada accepted that Bendectin was withdrawn for reasons associated with the litigation. According to the Toronto Star in 1989, Duchesnay “was required only to show it was manufacturing the same compound.”
By 1989, Diclectin had become a political hot potato, with both Liberal and NDP MPs putting pressure on the government to review the drug in light of media reports that Canadian lawyers were reviewing hundreds of birth-defect cases. Again, the focus was safety, not efficacy: NDP MP Neil Young demanded Diclectin be pulled from the market until “such time as it is proven to be safe for use.”
It was at this point that Koren became an outspoken defender, calling the prospect of a Diclectin recall “the saddest event in medical history,” and a “lynch mob run by lawyers” in a 1989 Toronto Star interview. “If Canada is the only country left in the world selling the drug, then that’s a good mark for us,” he said.
Koren would be one of four doctors who formed a 1989 Health Canada “advisory panel” to assess Diclectin. The panel was hastily convened to provide external political cover for the minister, recalls clinical pediatric pharmacologist Michele Brill-Edwards, then a senior medical adviser for Health Canada’s prescription drugs unit, who oversaw the meeting. “The goal was to determine if there was reliable evidence of teratogenicity.”
According to Health Canada, the group “reviewed human epidemiologic studies, animal studies and in vitro studies about Diclectin, and determined that it did not increase the risk of malformations.” Another member of the panel, American doctor Robert Brent, who acted as an expert witness for Merrell Dow in Bendectin cases and is a defender of the drug, summarized the data for the group. The panel concluded there was no reliable evidence linking the drug to birth defects, Brill-Edwards remembers. Brent and Brill-Edwards both told Maclean’s that Diclectin’s efficacy was not discussed.
Assurances about Diclectin continued from a steady stream of Motherisk papers, including the dramatically titled 1995 editorial, “Bendectin/Diclectin for morning sickness: A Canadian follow-up of an American tragedy,” co-authored by Koren, which claimed, “It would be a disservice to Canadian women to withdraw this drug from the market.”
By 1995, Koren’s national influence in the field of pediatric pharmacology was unparallelled. Motherisk offered a vital, and heretofore ignored service for doctors and pregnant women who needed information about drugs at a time when the topic was verboten. A genial man, Koren became the media’s go-to guy for stories involving drugs and pregnancy. He also held unique authority: He was the editor of numerous medical journals, a contributor to a monthly column in Canadian Family Physician and a prolific researcher renowned as a clinical-trial rainmaker for the hospital.
It demonstrates Koren’s value to SickKids that he retained his position after being implicated in a scandal in the late 1990s that would have ended the careers of most. This arose after hematologist Nancy Olivieri went public with what she believed to be serious side effects in a drug that treated a childhood blood disorder she was researching with Koren that was sponsored by drugmaker Apotex; Koren sent out vicious, anonymous letters to discredit Olivieri, then denied doing so until presented with DNA evidence. An investigation revealed that Koren put his name on reports drafted and co-authored by Apotex-funded researchers who used Olivieri’s data, but didn’t mention risks she’d identified. He also gave incorrect and false testimony against Olivieri, and failed to disclose a $250,000 “miscellaneous” grant from Apotex. Koren faced disciplinary action, including a six-month suspension (four with pay), but he kept his Motherisk position.
Koren took on the role of ardent champion of pregnant women. At a 2006 news conference sponsored by Duchesnay, Koren implored the federal government to help expectant mothers obtain easier access to some prescription drugs: “It’s very sad that, at the most vulnerable time of their lives, Canadian women are by themselves,” he said. “That’s not acceptable.”
For decades, Koren, Motherisk and Duchesnay worked together to raise awareness of NVP—and, as a result, awareness of the only approved remedy to treat it. Canada remains the only country to recommend prescription pyridoxine-doxylamine as a front-line NVP treatment. The American College of Obstetricians and Gynecologists first recommends over-the-counter vitamin B6. Britain’s National Institute for Health and Care Excellence advises ginger, accupressure and antihistamines if women ask for treatment.
Duchesnay partially sponsored Motherisk’s “morning sickness hotline,” which dispensed advice to pregnant women suffering from NVP, and also sponsored Motherisk’s first international meeting dedicated to NVP in 1998. A search for “Koren and Diclectin” in the medical research archive PubMed, which dates to 1995, reveals 28 articles, some Duchesnay-sponsored, all offering reassurances about the drug’s safety.
Some of Koren’s Duchesnay-sponsored research was aimed at broadening the audience for Diclectin: A 59-woman clinical trial conducted by Koren assessed the drug’s potential to prevent NVP, meaning symptom-free women could be prescribed the drug. The trial’s results, published in 2013, stated that “the pre-emptive use of Diclectin mitigated symptoms of severe NVP.”
The messaging was effective: In 1989, less than three per cent of pregnant women took the drug, according to the Globe and Mail at the time; today, it’s 50 per cent.
Koren was also instrumental in Duchesnay’s decade-long quest for FDA approval of pyridoxine-doxylamine in the U.S. Needing efficacy data, Duchesnay financially supported a 256-person clinical trial. Koren was the first author of seven summarizing the trial in a 2010 report that concluded the drug is “effective and well-tolerated” in treating NVP; the study also made clear that, before this, “no randomized control trial has evaluated the effectiveness of this new formulation of doxylamine succinate-pyridoxine hydrochloride for NVP,” and that the “only previously randomized studies were conducted with Bendectin 40 years ago, and were never published in peer-reviewed literature, but only submitted to the FDA.”
The FDA approved Diclegis in April 2013, giving it a “pregnancy category A rating,” its highest class of safety. Motherisk marked the occasion in a July 2013 review of the drug with the celebratory subtitle: “A new morning for American women.” A November 2013 booklet, How to Survive Morning Sickness Successfully, co-authored by Koren, named Diclectin and Diclegis “the medication of choice for morning sickness.” In a 2013 interview with Maclean’s, Koren spoke of his efforts to make the drug available in Israel; it was approved in that country in March 2015.
It is a measure of the authority held by Motherisk and Koren that St. Michael’s Diclectin research had so little traction. The researchers had hoped that the flawed 1997 meta-analysis would be retracted from the scientific literature since its conclusions are not supporting by its findings. That didn’t happen. They also failed in their bid for a change in prescription guidelines for treating morning sickness. The Society of Obstetricians and Gynaecologists announced that it examined the researchers’ concerns and determined that no change was needed in its guidelines that names Diclectin “the standard of care, since it has the greatest evidence to support its efficacy and safety.” The society, which counts Duchesnay as a sponsor, issued a brief online statement saying the drug was “safe.” (Maclean’s requests for an interview with the society went unanswered.)
Koren also dismissed the St. Michael’s researchers. While acknowledging some errors in Motherisk’s metastudy, he rejected the suggestion that treatment guidelines should change, for fear of upsetting women: “It’s very important not to put a lot of women at unease because of re-analysis and so forth,” he told the National Post.
Koren’s authority began to falter, however, with the detection of grievous errors in Motherisk’s drug and alcohol hair-testing program. Last year, the province of Ontario appointed an independent review of Motherisk practices, due in December; the review was significantly expanded this spring as questions were being asked about Koren’s financial relationship with Duchesnay. Koren was removed from “medical oversight” of the Motherisk lab program in March. He retired quietly in June.
Among medical researchers and academics, the financial relationship between Koren and Duchesnay had been the subject of concern for some time. Industry funding of medical research is common, but Motherisk’s role in counselling pregnant women raised worries about potential conflict of interest. In 2001, when it was announced that Koren was to receive the “Duchesnay and Canadian Foundation for Women’s Health Chair in better pharmacotherapy during pregnancy and lactation” at the University of Toronto, there was so much protest, the nomination was withdrawn. At issue was the incorrect perception that the Duchesnay-created chair was an official academic appointment; there was also outrage over Koren being honoured so soon after the Apotex scandal.
Koren, who did not respond to Maclean’s request for an interview, has been steadfast that the funding relationship did not compromise the integrity of his research: In 2013, he told the National Post that his group kept a strict arm’s-length relationship with industry donors.
The hospital is conducting its own internal review, which includes assessing the integrity of the 1997 Diclectin meta-analysis, hospital spokeswoman Matet Nebres told Maclean’s in an email. It found “no evidence that data was fabricated or falsified, but it did find “errors made in the citation of references.” It also identified fewer subjects than the more than 200,000 women referenced in the paper. The hospital hasn’t reviewed the accuracy of the study’s safety claim. Last week, SickKids announced it is “asking an expert in the field to conduct an independent re-analysis of the original data.”
In dealing with the crisis, the hospital has adopted a new candour and fuller disclosure, even before last week’s apology and report. It is forthcoming about Duchesnay’s donations to SickKids, which began in 1995, Nebres told Maclean’s: In total, “from 2000 to 2013, Duchesnay has provided $4.3 million in research funding.” All but “one small donation of $2,500 went to Motherisk program activity,” she wrote.
The following remain unknown: how much money Koren received from Duchesnay; how much he funnelled through the Research Leadership for Better Pharmacotherapy during Pregnancy and Lactation; and the date he became a paid consultant. A SickKids 2002 research disclosure obtained by Maclean’s reports that, between 1994 and 2002, Koren received $240,000 from the company.
Duchesnay itself reports new research in the works. This week, spokesman Ron Vaillancourt told Maclean’s that, due to “Dr. Persaud’s recent media outreach,” the six researchers who co-authored the 2010 clinical trial study with Koren are reassessing their work: They “feel so strongly about their initial findings that they have decided to re-analyze the data of the 2010 Diclectin efficacy study,” he said in an email. “Their conclusions are expected to be published in a peer-review journal.”
Meanwhile, Persaud finds himself in a Catch-22. On one hand, he has a motherlode of information about Diclectin, yet the gag order on him means he’s bound to silence. He’s better informed, but unable to speak. His quest for information about the drug has bordered on farce. It took him 3½ years to receive a response to his Access to Information request filed in September 2011. Months later, Health Canada emailed to say it was negotiating with a third party—Duchesnay—to decide which records they wanted to protect. Of the 359 pages Persaud received, 212 were censored, with other pages blacked-out, including information under “adverse events,” withheld because it violated “confidential trade secrets.” He has yet to receive all of the information from the access to information request.
Persaud is currently trying to unearth an unpublished 1975 Merrell Dow study into Bendectin’s efficacy conducted before the drug was reformulated. He has also asked Health Canada to extend the confidentiality agreement to include a statistician who can help analyze the data, he told Maclean’s. He realizes the quest is quixotic: The findings can’t be published in a peer-reviewed journal, because he can’t submit underlying research.
Persaud says he has been approached by lawyers who maintain the confidentiality agreement is inconsistent with Vanessa’s Law, and he could have grounds to sue Health Canada. He’s reviewing his options while remaining undaunted: “Women need to have full disclosure about the intersection of the legal and the clinical history of the drug,” he says. “Then they can come to an informed decision.” That won’t happen until Health Canada, or Duchesnay, makes public that raw data. Until then, both women and doctors will remain, unforgivably, in the dark.
Clarifications: The article has been amended to reflect the fact that “Diclectin is prescribed in one of two births” in Canada rather than to one of two pregnant women.
Dr. Nav Persaud and the St. Michael’s research team did not directly request that the 1997 Motherisk metastudy be removed from the medical literature. They thought that it might be retracted since its conclusions are not supporting by its findings.
Dr. Persaud has yet to receive all of the information from his 2011 Freedom of Information request regarding Diclectin.